Direct reprogramming for targeted medicine (DiRECT)
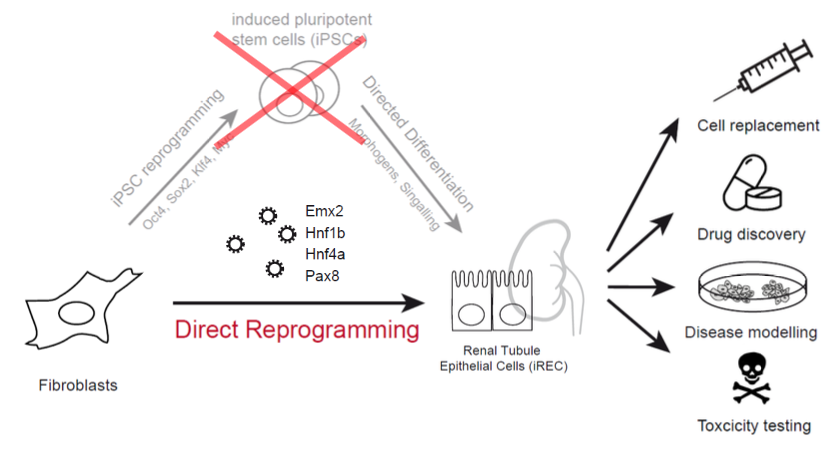
Direct reprogramming refers to the conversion of one cell type into another. We developed a method to transform fibroblasts into induced renal tubular epithelial-like cells (iRECs). This conversion process requires three to four transcription factors, and does not require the use or production of pluripotent cells.
We are currently using this technology to develop novel in vitro models for a number of renal diseases.
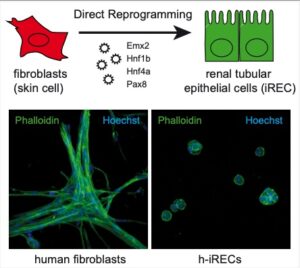
iRECs have striking similarities to native renal tubular cells. They form epithelial spheroids that are correctly polarized in 3D culture and self assemble when seeded into an empty kidney ECM scaffold. They express a large number of tubular enriched proteins and proliferate in culture. They can be passaged without loosing their differentiation, which makes it possible to derive disease specific reprogrammed cells and expand them for various usages.
read more here:
Kaminski M, Tosic J, Kresbach C, Engel H, Klockenbusche J, Müller A-L, Pichler R, Grahammer F, Kretz O, Huber TB, Walz G, Arnold SJ**, Lienkamp SS**. (2016) Direct Reprogramming of Fibroblasts into Renal Tubular Epithelial Cells by Defined Transcription Factors. Nature Cell Biology 18, 1269-1280
download pdf here.
The DiRECT project aims to
-
- Clarify the mechanisms behind direct reprogramming to iRECs
- Identify novel routes to reprogramming
- Optimize reprogramming to segment specific cell identities
- Establish in vitro models for human renal diseases
In collaboration with the Institute of Microsystems Technology (IMTEK) at the University of Freiburg in Germany, we established a method that utilizes bioprinting and the self-organizing capacities of iRECs to form perfusable tubules in vitro.
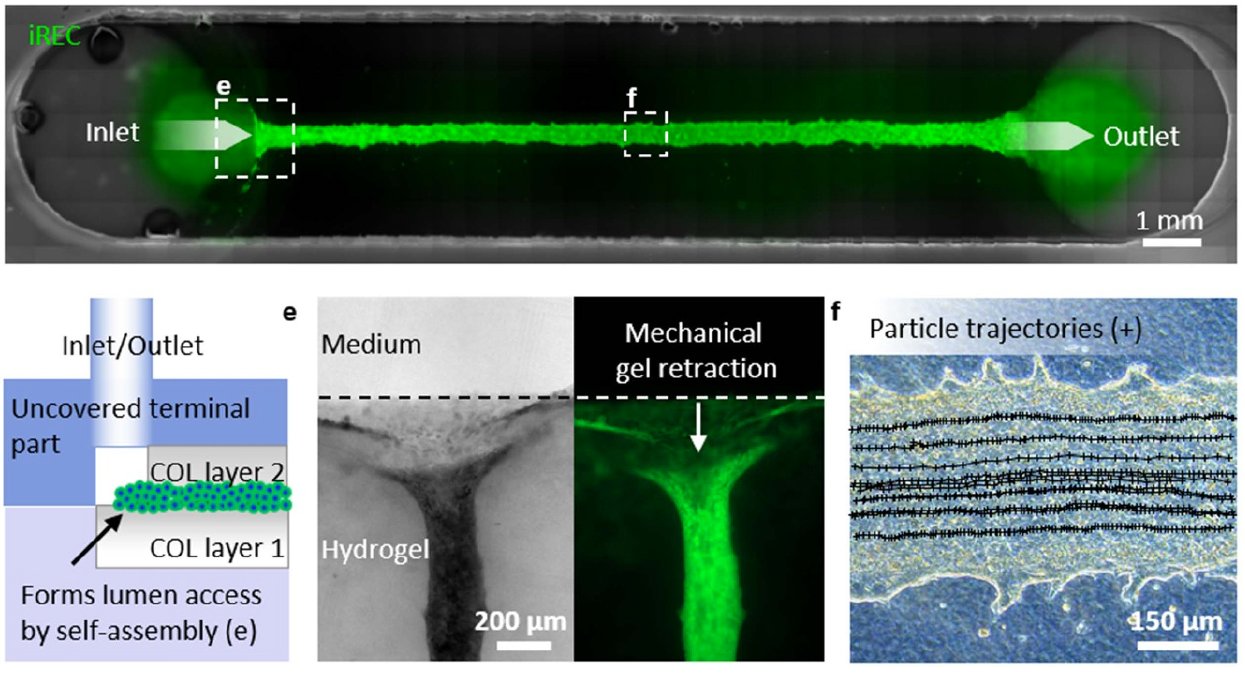
Publication:
Troendle, K., Rizzo, L., Pichler, R., Koch, F., Itani, A., Zengerle, R., Lienkamp, SS., Koltay, P., & Zimmermann, S. (2021). Scalable fabrication of renal spheroids and nephron-like tubules by bioprinting and controlled self-assembly of epithelial cells. Biofabrication
download pdf here.
In collaboration with the Institute imagine in Paris (Prof. Simons) we interrogated the effects of a patient specific mutation in HNF4A using iRECs in a model for Renal Fanconi Syndrome:
Marchesin, V., Pérez-Martí, A., Le Meur, G., Pichler, R., Grand, K., Klootwijk, E. D., Kesselheim, A., Kleta, R., Lienkamp, S., & Simons, M. (2019). Molecular Basis for Autosomal-Dominant Renal Fanconi Syndrome Caused by HNF4A. Cell Reports, 29(13), 4407-4421.e5.
download pdf here.
This ongoing project is generously funded by the the European Union’s Horizon 2020 research and innovation programme (StrGrant)
As a member of the National Centre for Competence in Research Kidney.CH, we make this technology available to consortium members and other interested groups.